Why Is Trust Stifling Our RBM Implementations?

By Dan Schell, Chief Editor, Clinical Leader

In the words of Billy Joel, when it comes to using risk-based monitoring (RBM), it’s a matter of trust. I know, I know … that’s a cheesy way to segue into a discussion about a clinical operations topic, but that concept of trust kept coming up in my research for this article. And, I like Billy Joel.
It all started when I was sent the Bourne Partners Clinical Trial Technologies Market Research Report, January 22, 2025. The section on RBM intrigued me. We all know the use of this approach has been increasing (especially since Covid), and its potential to save money and time is undeniable. For example, the Bourne report states, “In our view, traditional site monitoring with on-site CRAs can account for as much as 25%-30% of a typical Phase 3 trial. With the median clinical trial costing $9.4 million, total site monitoring related costs over the course of a trial might equate to upwards of $2 million. So, reduced CRA travel/site visits could comfortably save multiple hundreds of thousands of dollars per trial.” Sounds like a win-win, right? Those bleary-eyed road warrior CRAs get a break, we’re proactively assessing any safety and quality issues, and we’re saving money. But it’s that pesky trust issue that’s the catch. A lot of ClinOps folks are cool with implementing pieces of RBM like central monitoring or initial risk assessments, but when it comes to agreeing to anything less than 100% SDV (source data verification) or SDR (source data review), trepidation creeps in.
More Than Just A Checkbox
I knew I needed more info on this topic to really understand not only its trajectory, but the nuances associated with its adoption. Coincidentally, I had just finished a conversation with TransCelerate CEO Janice Chang who had given me a big-picture overview of their initiatives and goals. As their RBM initiative had been completed in 2021, I turned to ACRO, which had released in early 2024 its fifth annual report/survey on RBM and RBQM adoption in clinical trials.
The report leads off with an impressive stat: “In 2023, 88% of clinical trials had at least one RBM or RBQM component included, a massive improvement from 2019, when this figure was only 53%.” It also had some really interesting charts such as the ones I’ve included below about the adoption of RBM-related components both in ongoing and new studies.
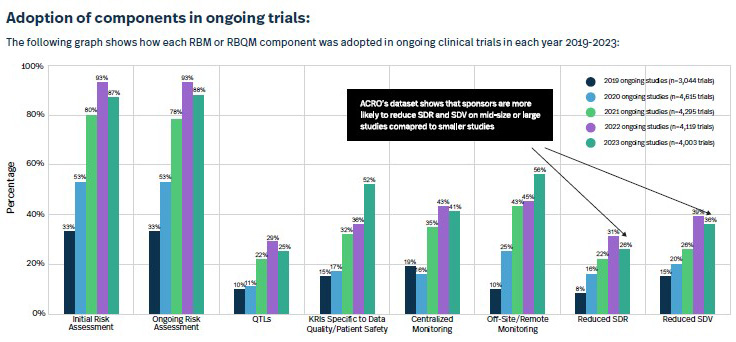
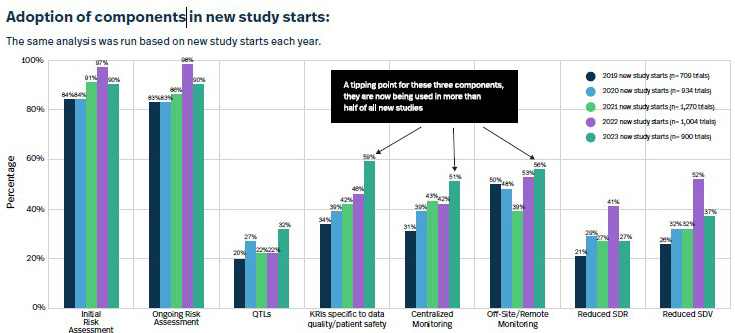
Luckily, I was able to connect with ACRO member Nicole Stansbury, who’s day job is SVP, global clinical operations at Premier Research. With over 30 years experience in clinical trials, Stansbury remembers the days — prior to 2006 or so — when clinical monitoring plans were only adapted maybe once or twice in a five-year study. “It became more and more obvious that this should not be a one-time exercise; there should be ongoing risk assessments,” she says. “We wanted to make sure that as we’re building our functional plans, monitoring strategy, data-management plans, and medical-monitoring strategy, we are doing our best to mitigate the risks we could anticipate at that point in time. It was more about the maturity of the entire process as opposed to simply a checkbox.”
A Corn-y RBM Cost Analogy
There’s no doubt RBM processes — and just the use of RBM components — have matured in recent years. So, too, have perceptions surrounding the cost savings related to RBM.
In 2019, my predecessor, Ed Miseta, interviewed Jennifer Newman, global project leader, Regulatory Affairs/Clinical Operations for Celldex Therapeutics, who had been part of a huge RBM implementation. Newman commented that the RBM initiative didn’t yield the large-scale cost savings initially predicted. “I think realistically, you can reduce the cost of on-site monitoring using central monitoring and a risk-based approach,” she said. “But, I think it’s more in an order of magnitude of 10%, and that may be [approximately] what you’ve paid to implement it.”
Stansbury insists that the cost savings shouldn’t be measured by one or two RBM components, but instead, from how all those pieces work together. “I would say that most companies conducting a small study can save maybe 5%-10% off their total monitoring costs even after adding in technology fees. For larger trials like a Phase 3, if you really push the boundaries and trust the process and use all the pieces in the ‘right’ amount, you are going to see anywhere from 15% to 25% cost savings, which can equate to millions of dollars.”
The ACRO report indicates that acceptance of RBM components is on the rise, with more than half of the ongoing clinical trials incorporating risk assessments, KRIs (key risk indicators), and off-site remote monitoring. Still, the industry is missing out on time and costs savings by not embracing anything less than 100% SDV/SDR. Stansbury has a somewhat unconventional yet very effective analogy she uses to explain the downside to not embracing all aspects of the RBM puzzle.
It involves corn.
Stay with me on this.
In her analogy, a kernel of corn is a data point, and a row of corn on a cob is a procedure. The corn cob is a patient visit. The patient is a stalk of corn, and the site is a row of corn in the field. Finally, the entire study is this giant field of corn. Can you picture all of that?
“When we’re asking people to do 100% SDR and SDV, what we’re essentially asking them to do is send 20 CRAs into this cornfield and have them inspect every kernel on every cob,” explains Stansbury. “How is that efficient?”
Now imagine a drone flying above that field. It can identify things such as brown sections on corn stalks or malformed/very small corn cobs. This is the equivalent of central monitoring. With this information, you can pinpoint problem areas and deploy just the right amount of CRAs to inspect specific rows or stalks. Perhaps they find that the soil was dried out or that there was some mold growing around the stalk or even some kind of pest or worm causing the problem. Using that data can help you proactively adapt and make a change that could impact overall data quality or even safety — without having to inspect every kernel. It’s not 100% SDV/SDR, it’s a sampling. “That’s how you get higher quality data at a fraction of the cost,” she says.
Interestingly, Stansbury adds that in the past it would be the medical monitors who were resistant to this approach, but today, they’re on board. It’s the ops folks who fear anything going wrong and are only comfortable with that 100% SDV/SDR. But she brings up a good point about that age-old adage in drug development about failing fast. Would you rather spend all that time and money analyzing millions of unnecessary data points, or would you rather use something like central monitoring to uncover a problem much sooner in the trial process?
Those cost savings offer another advantage, too — maybe you can launch more studies. “Say you’re a Big Pharma running 250 trials a year,” posits Stansbury. “Do the math. If, through RBM, you can save 25% on your Phase 3 studies and maybe 5%-10% on your Phase 1 and 2 studies, then maybe you can pull two more products off the shelf that previously didn’t have a budget to develop. If you do it right across the 250 trials, maybe you have budget to add some other products into your pipeline instead of waiting five years.”
So, the metrics are there; it’s just the trust that we are still lacking.
