What Drives eConsent Adoption? Reasons May Be Different Than You Think
By Jeanie Magdalena Gatewood, principal, Gatewood Global

In recent years, the FDA has issued updated guidance related to electronic informed consent (eConsent) technologies, signaling a significant shift from traditional paper-based processes. This transition to digital formats aims to enhance the efficiency of clinical trials, improve patient engagement, and ensure clearer comprehension of consent documentation by participants (Buckley et. al. 2022). The rapid revolution of clinical trial operations, exemplified by the adoption of digital technologies in general, compels a critical reassessment of priorities within the field.
A recent LinkedIn poll, conducted by one of my colleagues and distributed among clinical research professionals, revealed a striking emphasis on efficiency and cost reduction. Thirty-eight percent of respondents identified these factors as the highest drivers for implementing an eConsent process for their clinical trials. Participant comprehension, surprisingly, trailed at 27%. However, the most startling insight gained is that these focal points surpass both the traditionally paramount concerns of protocol compliance and patient safety, which garnered only 22% and 14%, respectively.
This prioritization raises pertinent questions about the future landscape of clinical trials: Are we, as a profession, willing to sacrifice essential ethical standards for operational efficiency? And, how can eConsent tools be optimized to enhance both efficiency and patient-centric outcomes? These questions formed the basis of a comprehensive survey aimed at understanding the underlying drivers and deterrents of eConsent adoption among clinical research professionals. The findings suggest a complex interplay between the pursuit of efficiency and the imperative to uphold the integrity and safety of clinical trials.
Delving Deeper Into eConsent Dynamics
To further our understanding of the factors influencing the adoption of eConsent, as part of my studies as a student researcher enrolled in the Drexel LeBow College of Business, Executive Doctorate in Business (DBA) Program, I meticulously designed and administered a structured survey. The survey design carefully considered response strategies, as suboptimal design can lead to “satisficing,” where respondents provide satisfactory but potentially non-optimal answers due to cognitive overload (Krosnick, 1991). This survey comprised a mix of qualitative and quantitative prompts, ranging from multiple choice to slider scales and constant sum questions, allowing for a comprehensive assessment of respondents’ perspectives on various aspects of eConsent. Questions covered topics from general adoption decisions such as efficiency, cost compliance, and participant understanding.
I distributed the survey via a multichannel approach to ensure a diverse and representative sample of clinical research professionals. Channels included a LinkedIn Live webinar, direct email, specialized survey networks, and an invitation to over 5,000 contacts within my professional network, aimed at maximizing reach and participation. To encourage honest and thoughtful responses, the survey was designed to be completely anonymous, with no incentives offered to respondents. This anonymity was critical in eliciting responses that reflected genuine perceptions and experiences without any bias induced by compensation or recognition.
A Complex Landscape Of Adoption Drivers
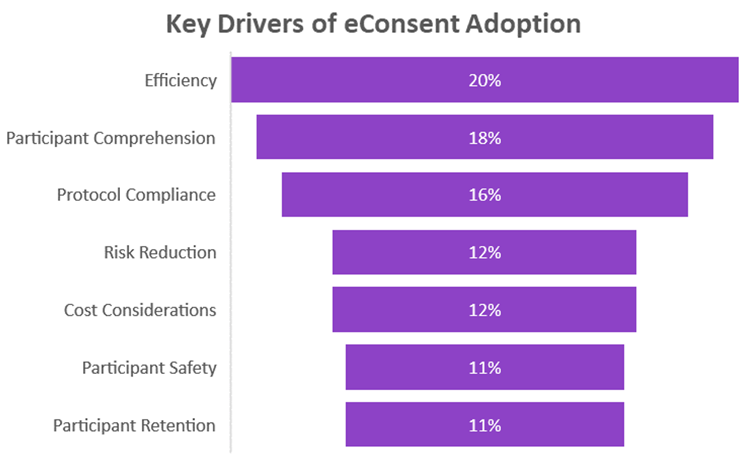
The survey revealed that while efficiency and cost containment are indeed significant drivers for adopting eConsent platforms, other factors such as compliance risk reduction and participant comprehension are equally crucial. Notably:
Broad Adoption: Seventy-five percent of respondents indicated their organizations had either fully or partially adopted eConsent technologies.
Diverse Motivations: While the initial poll highlighted efficiency as a primary driver, our survey respondents painted a more nuanced picture where the interplay of compliance, safety, and comprehension also play critical roles.
Surprising Disconnects: Many respondents, despite attaining significantly powerful professional roles, reported having minimal input into discussions and determinations related to technology adoption within their organizations. This highlights a potential gap in organizational decision-making processes.
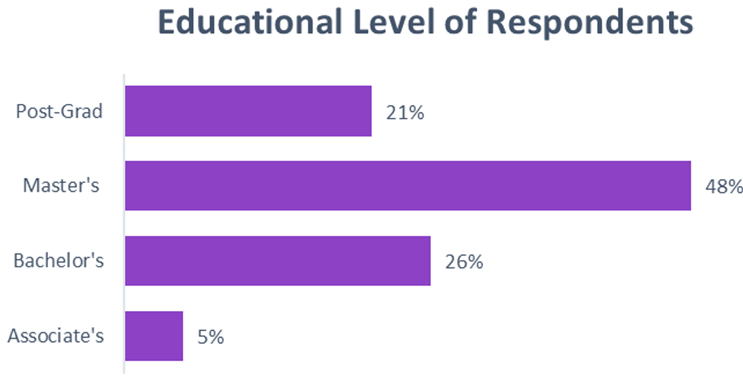
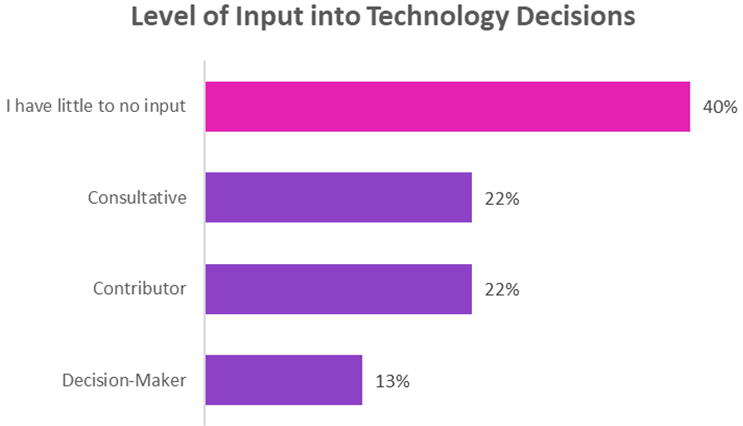
The application of statistical tests, including chi-squared and t-tests, helped validate the significance of the findings. For instance, no significant relationship was found between the relatively high levels of education or years of experience as a clinical research professional and the degree of input into technology decisions. Again, this is a surprising insight given the respondents’ high qualifications.
Implications For Practice
These findings bring forward the need for a balanced approach to the adoption of eConsent technology. While striving for efficiency and cost-effectiveness, it is imperative that organizations involved in clinical research also prioritize participant comprehension, safety, and compliance to maintain the integrity of a truly informed, technologically sound consent process. With key drivers and deterrents identified, and based on the findings of this survey, we can formulate enhanced targeted strategies.
Enhance Trust and Validate Innovation: Trust in technology and openness to innovation were noted as significant factors influencing the adoption of eConsent (Gefen et. al. 2003). These elements suggest a readiness to embrace new technologies if they are proven trustworthy and if they clearly innovate on existing processes. Given the importance of trust in technology, organizations engaged in clinical research should present validation studies to demonstrate the reliability, security, and user-friendliness of eConsent platforms.
General Acceptance of eConsent: A significant portion of respondents indicated a strong inclination toward the adoption of eConsent, reflecting broad acceptance across the industry. This suggests that the majority of the community sees the potential benefits of eConsent outweighing the challenges. Regular monitoring of usage and satisfaction levels with eConsent tools through feedback surveys and user forums can help iteratively improve the technology and address any emerging issues before they become widespread concerns (Zaki et. al. 2024).
Challenges in Decision-Making and Technology Acceptance: Despite adoption, there was a notable concern about the lack of inclusion of diverse stakeholder groups in the decision-making process, particularly those directly involved in clinical trials who may not have substantial input yet serve as the responsible party or end user (Joseph and Gaba, 2020). Addressing the challenge of limited input in technology adoption by the establishment of advisory panels that include a broad range of stakeholders, including junior staff, senior researchers, and IT specialists will ensure that the eConsent solutions implemented are well-rounded and address the needs of all users.
The Future Of eConsent
The findings from my survey underscore a pivotal moment in the evolution of clinical research operational practices, marked by a discernable shift toward eConsent technologies. The potential for eConsent to streamline processes, reduce costs, and enhance participant engagement is clear. However, the implementation landscape is nuanced, revealing that such technologies are not only about efficiency but also about enhancing the integrity and quality of clinical trial consent.
Despite the enthusiasm for digital solutions, my research has identified significant gaps in stakeholder involvement in decision-making. Many professionals, particularly those with considerable experience and qualifications, report feeling sidelined in company determinations about technology adoption. This disconnect could undermine the potential benefits of eConsent by limiting its effective and ethical integration into practice (Chow et. al., 2023).
Strategic Recommendations
- Enhance Stakeholder Engagement: Clinical research sponsors, CROs, and sites should develop strategies to involve a broader range of stakeholders, including those with direct clinical and research responsibilities, in technology decision-making processes (Tang et. al. 2022).
- Develop Tailored Communication Strategies: Tailored strategies to communicate the benefits and challenges of eConsent should be developed to address the specific concerns and needs of various stakeholder groups within the clinical research community.
- Implement Adaptive Training Programs: To maximize the impact and utilization of eConsent, organizations should invest in adaptive training programs that are responsive to the evolving needs and feedback of all users, thereby improving both the acceptance and effectiveness of these technologies (Xu et. al. 2019).
Areas For Future Research
- Decision-Making Processes: Further research is required to explore how decisions about technology adoption are made within clinical research organizations and how these processes can be made more inclusive.
- Comprehensive Impact Assessment: Studies should examine the long-term impacts of eConsent on trial efficiency, participant satisfaction, and overall trial integrity, beyond the initial implementation phase.
- Training and Support Structures: Investigating the effectiveness of current training programs for eConsent technologies could identify gaps and opportunities for enhancing user competency and acceptance.
Pioneering Potential
Finally, I’ll leave you with this future vision to consider. As we explore the evolving landscape of eConsent in clinical research, the next frontier involves the integration of AI. This promising advancement could transform eConsent completely into a dynamic, personalized experience that adapts to individual participant needs in real time. Imagine a system that not only informs but also interacts, connecting comprehension and compliance through intelligent algorithms (Saroglu et. al., 2024).
While the full potential of AI-enhanced eConsent will be detailed in my forthcoming publication, it is clear that such innovations could significantly refine how consent is obtained and maintained across global trials. This is not solely about technological enhancements but about redefining participant engagement and ethical standards in clinical research. Stay tuned for an in-depth exploration of how AI could revolutionize the eConsent process, making it more intuitive, interesting, and perfectly participant-focused.
References:
Buckley, M. T., O’Shea, M.R., Kundu, S., Lipitz-Snyderman, A., Kuperman, G., Shah, S., Iasonos, A., Houston, C., Terzulli, S. L., Lengfellner, J. M., & Sabbatini, P. (2022). Digitalizing the clinical research informed consent process: Assessing the participant experience in comparison with traditional paper-based methods. JCO Oncology Practice, 19, e355-e364. https://doi.org/10.1200/OP.22.00425
Chow, J., Ong, T., Morgan, A., & Khanna, S. (2023). The use of wearable devices in oncology patients: Emerging trends and clinical implications. Journal of Clinical Oncology, 41(11), 1572-1584. https://doi.org/10/1200/JCO.2023.41.11.1572
Gefen, D., Karahanna, E., & Straub, D. W. (2003). Trust and TAM in online shopping: An integrated model. MIS Quarterly, 27(1), 51-90. https://www.proquest.com/scholarly-jounals/trust-tam-online-shopping-integrated-model/docview/218117684/se-2
Joseph, J., & Gaba, A. (2020). Organizational structure and decision-making: An exploratory analysis. Academy of Management Annals, 14(2), 789-813. https://doi.org/10.5465?annals.2020.0056
Krosnick, J. A. (1991). Response strategies for coping with the cognitive demands of attitude measures in surveys. Applied Cognitive Psychology, 5(3), 213-236.
Saroglu, H.E., Shayea, I., Saoud, B., Azmi, M. H., El-Saleh, A. A., Saad, S. A., & Alnakhli, M. (2024). Machine learning IoT and 5G technologies for breast cancer studies: A review. Alexandria Engineering Journal, 89, 210-223. https://doi.org/10.1016/j.aeg.2024.01.043
Tang, P. M., Koopman, J., McClean, S. T., Zhang, J. H., Li, C. H., De Cremer, D., Lu, Y., & Ng, C. T. S. (2022/06//). When conscientious employees meet intelligent machines: An integrative approach inspired by complementarity theory and role theory. Academy of Management Journal, 65(3), 1019. https://doi.org/10.5465/amj.2020.1516
Xu, J., Yang, P., Xue, S., Sharma, B., Sanchez-Martin, M., Wang, F., Beaty, K. A., Dehan, E., & Parikh, B. (2019). Translating cancer genomics into precision medicine with artificial intelligence: Applications, challenges, and future perspectives. Human Genetics, 138(1), 109-124. https://doi.org/10/1007/s00439-019-01970-5
Zaki, H., Shayea, I., Saoud, B., Azmi, M. H., El-saleh, A. A., Saad, S. A., & Alankhi, M. (2024). Advancing patient outcomes: The intersection of biomedical engineering, medical devices, and AI. EasyChair Preprints, 12174.
Acknowledgments:
The author wishes to express sincere gratitude to the faculty and colleagues of the Drexel LeBow College of Business DBA Program, Cohort 7, for their invaluable support and guidance throughout this research. Special thanks are also extended to all the clinical research professionals and participants who generously shared their time and insights by contributing to the survey.
Additionally, the author is profoundly grateful to John H. Krantz, Ph.D., professor at Hanover College, whose platform (Experiments in Psychology) facilitated the administration of the survey. His assistance was crucial in enabling the successful collection of data and the broader dissemination of this work.
About The Author:
 Jeanie is a doctoral student and the principal of Gatewood Global — a boutique clinical research solutions company. In biotech and academic research, she is an accomplished and committed health-tech strategist driving innovation, trendsetting, and transforming clinical investigations via emerging technologies. As an expert member of the GLG Network, Jeanie provides investor insights and acts as an asset and acquisition advisor. Through The Medical Affairs Company, she has contracted with Carisma Therapeutics for their first-in-human studies. Jeanie is a thought leader with a portfolio of 70+ publications, presentations, and programs to her credit. She has represented companies and technologies including Antares Genetics and BD BIoSciences. Bringing 25+ years of direct clinical experience as an oncology RN and 20+ years in clinical research to her clientele, she’s crafted a career at the VP & director level at IQVIA, Inteliquet, Fox Chase Cancer Center, NYU Langone Medical Center, and AstraZeneca’s Aptium.
Jeanie is a doctoral student and the principal of Gatewood Global — a boutique clinical research solutions company. In biotech and academic research, she is an accomplished and committed health-tech strategist driving innovation, trendsetting, and transforming clinical investigations via emerging technologies. As an expert member of the GLG Network, Jeanie provides investor insights and acts as an asset and acquisition advisor. Through The Medical Affairs Company, she has contracted with Carisma Therapeutics for their first-in-human studies. Jeanie is a thought leader with a portfolio of 70+ publications, presentations, and programs to her credit. She has represented companies and technologies including Antares Genetics and BD BIoSciences. Bringing 25+ years of direct clinical experience as an oncology RN and 20+ years in clinical research to her clientele, she’s crafted a career at the VP & director level at IQVIA, Inteliquet, Fox Chase Cancer Center, NYU Langone Medical Center, and AstraZeneca’s Aptium.
