From Supercomputers To The Cloud: How Pharma's R&D Infrastructure Transformation Impacts Clinical Trials
By Kannan Ramachandran, category specialist - cloud computing & data center, and Mathini Ilancheran, research manager, R&D, Beroe Inc.

In 2025, pharmaceutical R&D entered a new phase of AI-driven research. Leading companies announced large-scale investments in AI and high-performance computing (HPC) to accelerate drug discovery and development. These initiatives are not incremental upgrades, and they represent a fundamental shift in scientific infrastructure, impacting everything from molecular design to clinical trial execution.
On June 11, 2025, Novo Nordisk partnered with NVIDIA and the Danish Centre for AI Innovation (DCAI) to harness the Gefion sovereign AI supercomputer, powered by NVIDIA DGX SuperPOD technology.1 This platform enables generative and agentic AI models for early-stage research, including molecule design and single-cell simulations.
Just months later, on October 28, 2025, Eli Lilly announced a collaboration with NVIDIA to build the pharma industry’s first in-house AI factory, a DGX-based supercomputer equipped with Blackwell Ultra GPUs and running on 100% renewable energy.2 This system is designed for end-to-end AI workflows, from data ingestion and model training to inference, and integrates federated learning through Lilly’s TuneLab platform, enabling collaborative innovation with biotech partners.
Together, these partnerships mark a turning point: Pharma R&D is moving from traditional compute clusters to AI-driven HPC ecosystems and hybrid architectures that combine on-premise supercomputing with elastic cloud infrastructure. The implications extend beyond discovery to clinical data management, trial design, and operational analytics. For procurement leaders, this evolution demands new sourcing strategies to balance long-term HPC investments with cloud-based scalability, compliance, and cost optimization.
The Role of Cloud Computing in Clinical Development
While HPC platforms deliver the raw power needed for deep learning, molecular simulation, and generative AI, cloud computing provides the elasticity, scalability, and global reach essential for modern clinical trial operations. This convergence is not optional, and it’s becoming the backbone of data-driven drug development.
Market signals underscore this trend.
The clinical trials cloud computing market is estimated at $2.5 billion in 2025, projected to reach nearly $8 billion by 2033, at a CAGR of ~15%.3
Clinical Trials Cloud Computing Market (in $ Billions)
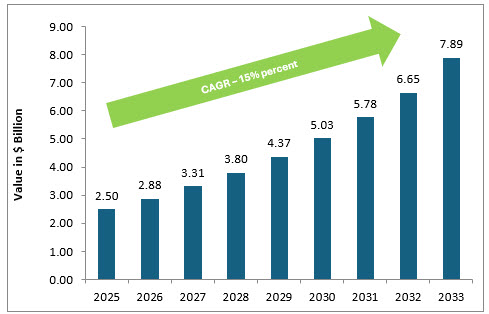
Source: Data Insight Market
The broader pharmaceutical cloud computing market was valued at $4.5 billion in 2023 and is forecast to grow to $15.2 billion by 2032, at a CAGR of ~14.5%.4
Pharmaceutical Trials Cloud Computing Market (in $ Billions)
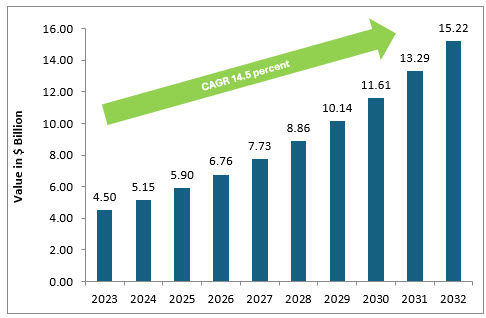
Source: Data Intelo
Why Cloud Matters for Pharma
To fully realize the benefits of AI-driven research, pharmaceutical companies are leveraging cloud computing to modernize clinical development. Beyond raw compute power, cloud platforms deliver capabilities that enable patient-centric trials, real-time insights, and regulatory compliance at scale. These capabilities represent the practical intersection of technology and clinical operations, making trials more agile and cost-efficient.
Cloud computing underpins these capabilities in clinical development. Here’s how it drives value across key areas:
- Decentralized Trials: Cloud platforms enable secure remote data capture and patient monitoring tools, reducing dependency on physical trial sites.
- Real-Time Data Integration: Cloud-based analytics aggregate and harmonize data streams from multiple geographies for faster insights.
- Regulatory Compliance: Validated cloud environments ensure adherence to GCP and 21 CFR Part 11 through built-in audit trails and encryption.
- Elastic Scalability: Cloud infrastructure scales dynamically to handle peak trial workloads without costly on-premises expansion.
Supercomputers like Gefion or Lilly’s DGX-based AI factory deliver concentrated compute power for model training and molecular design. In contrast, cloud systems complement these capabilities by scaling analytics, visualization, and data pipelines across the clinical value chain. Together, they form a hybrid architecture that integrates on-premises AI factories with regulated cloud environments, ensuring both performance and compliance. Table 1 below summarizes the functional roles of HPC and cloud in pharmaceutical R&D and their procurement implications.
Table 1: HPC and Cloud: The Converging Continuum
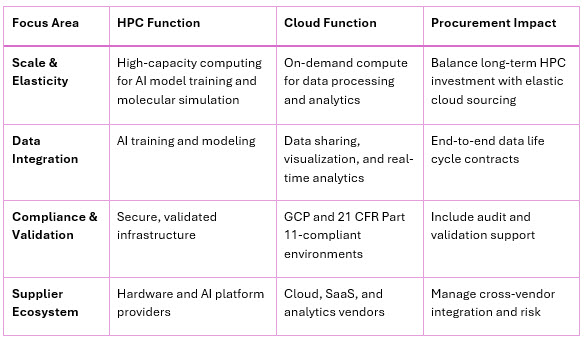
Source: Deloitte Life Sciences IT Infrastructure Report, 2025; AWS Pharma Cloud Documentation; NVIDIA AI Factory Case Studies.
Procurement Implications for Cloud in Clinical Trials
The convergence of AI-driven HPC and elastic cloud infrastructure is redefining sourcing strategies for pharmaceutical organizations. Procurement teams must move beyond traditional IT contracting and adopt frameworks that address hybrid architectures, regulatory compliance, and cost governance. Key imperatives include:
- Elastic Cloud Infrastructure
Source cloud platforms with clinical trial-specific service level agreements (SLAs), validated configurations, and compliance clauses aligned with GCP and 21 CFR Part 11. Ensure providers offer audit-ready environments for regulatory inspections.5 - Hybrid Compute Architecture
Design procurement models that integrate on-premise AI factories (for HPC-intensive workloads) with cloud-based workflows for trial data management, analytics, and visualization. This hybrid approach optimizes performance and scalability.6 - Vendor Ecosystem Collaboration
Negotiate joint contracts across HPC vendors, public cloud providers, and SaaS platforms for clinical trial management. Ensure interoperability and data life cycle continuity across multiple suppliers.7 - Compliance and Auditability
Embed data integrity checks, validation protocols, and regional compliance requirements into every sourcing agreement. Include provisions for continuous monitoring and real-time audit trails.8 - Cost Optimization
Implement financial operations governance models to track variable compute spending across HPC and cloud environments. Use predictive analytics to forecast usage and prevent cost overruns.9 - Data Sovereignty and Security
Incorporate regional hosting mandates, cross-border compliance clauses, and zero-trust security frameworks to safeguard sensitive trial data in global operations.10
IT Suppliers Pivoting Toward Pharma
Technology providers are rapidly reshaping portfolios to meet the growing demand for AI-driven drug discovery and digitally enabled clinical trials. For procurement leaders, understanding these shifts is critical to building resilient, compliant, and cost-effective sourcing strategies. Table 2 below summarizes recent strategic moves by key IT suppliers and their relevance for pharma procurement strategies.
Table 2: Strategic Initiatives by IT Suppliers and Their Procurement Implications
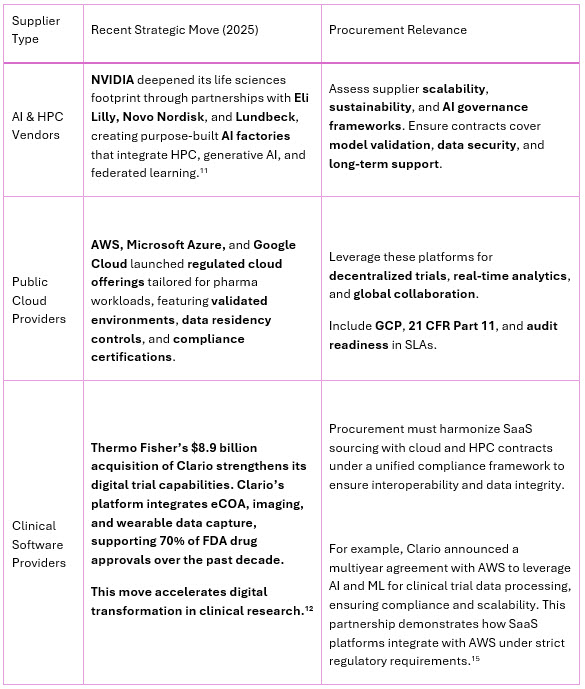
Source: NVIDIA Investor News; AWS Healthcare Solutions; Thermo Fisher Investor Relations; Clario Newsroom
Conclusion and Future Outlook
The 2025 collaborations between Eli Lilly, Novo Nordisk, and NVIDIA represent more than technology partnerships as they mark the beginning of a new scientific paradigm. By merging AI-driven HPC infrastructures with elastic cloud ecosystems, pharmaceutical R&D is evolving into an interconnected, data-centric model that accelerates discovery and transforms clinical development.
For procurement leaders, the mandate is clear:
- Connect on-premises AI factories with scalable cloud architectures to enable seamless data flow across research and trials. For example, Eli Lilly partnered with NVIDIA to build an AI supercomputer (“AI factory”) integrated with federated learning via TuneLab, while leveraging AWS for elastic trial data processing. This hybrid model accelerated molecule screening and reduced compute bottlenecks.12
- Ensure interoperability, compliance, and auditability in every sourcing decision. Clario’s multiyear agreement with AWS enabled integration of eCOA and remote monitoring tools into HIPAA-compliant cloud environments, ensuring GCP and 21 CFR Part 11 compliance with joint SLAs.13
- Adopt hybrid strategies that balance fixed HPC investments with flexible, cost-optimized cloud resources. For example, Bristol Myers Squibb used AWS for in silico trial simulations, reducing analysis time by 98% and cutting trial duration by nearly a year, while maintaining on-premise HPC for AI model training.14, 15
Cloud computing has moved from a supporting IT function to the core of scientific infrastructure, enabling decentralized trials, real-time analytics, and global collaboration. As AI-driven drug discovery matures, expect its impact on clinical trial design, patient recruitment, and operational analytics to accelerate, though regulatory timelines mean the full benefits will materialize closer to 2030.
The future of pharma is hybrid, intelligent, and collaborative, where AI factories and sovereign clouds work in tandem to deliver smarter trials and faster cures.
References:
- NVIDIA Corporation, “NVIDIA Partners With Novo Nordisk and DCAI to Advance Drug Discovery,” June 11, 2025. Available: https://investor.nvidia.com/news/press-release-details/2025/NVIDIA-Partners-With-Novo-Nordisk-and-DCAI-to-Advance-Drug-Discovery/default.aspx
- Reuters, “Lilly partners with Nvidia on AI supercomputer to speed up drug development,” Oct. 28, 2025. Available: https://www.reuters.com/business/healthcare-pharmaceuticals/lilly-partners-with-nvidia-ai-supercomputer-speed-up-drug-development-2025-10-28/
- Data Insights Market, “Cloud Computing in Clinical Trials Industry Growth Dynamics and Insights,” 2025. Available: https://www.datainsightsmarket.com/reports/cloud-computing-in-clinical-trials-497466
- Data Intelo, “Cloud Computing in Pharmaceutical Market,” 2023. Available: https://dataintelo.com/report/cloud-computing-in-pharmaceutical-market
- Pharma Connections, “Cloud Infrastructure Validation in the Pharmaceutical Industry,” April 2025. Available: https://pharmaconnections.in/cloud-infrastructure-validation-in-the-pharmaceutical-industry/
- IBM, “Case Studies Pfizer,” Available: https://www.ibm.com/case-studies/pfizer
- McKinsey, “Building a shared vision for Pharma R&D Supplier Partnerships,” Available: https://www.mckinsey.com/industries/life-sciences/our-insights/building-a-shared-vision-for-pharma-r-and-d-supplier-partnerships
- GMP, Cloud Computing content of a SLA contract with a XssS provider, Available: https://www.gmp-compliance.org/gmp-news/cloud-computing-content-of-a-sla-contract-with-a-xaas-provider
- Quinnox, FinOps best Practices, Available: https://www.quinnox.com/blogs/finops-best-practices/
- ACInfotech, Sovereign cloud healthcare AI companies, Available: https://www.aciinfotech.com/blogs/sovereign-cloud-healthcare-ai-compliance
- PharmaVoice, “AI giants Nvidia, Microsoft and Google are making critical moves in pharma R&D,” Oct. 22, 2025. Available: https://www.pharmavoice.com/news/pharma-nvidia-microsoft-google-research-drug-market-bubble/803407/
- Contract Pharma, “Thermo Fisher to Acquire Clario for $8.875 Billion,” Oct. 29, 2025. Available: https://www.contractpharma.com/breaking-news/thermo-fisher-to-acquire-clario-for-8-875-billion/
- Clario collaborates with AWS to push new boundaries in clinical data analysis with generative AI,” April 22, 2025, Available: https://clario.com/about/newsroom/clario-collaborates-with-aws-to-push-new-boundaries-in-clinical-data-analysis-with-generative-ai/
- Lilly partners with NVIDIA to build the industry's most powerful AI supercomputer, supercharging medicine discovery and delivery for patients, Oct. 28, 2025, Available: https://investor.lilly.com/news-releases/news-release-details/lilly-partners-nvidia-build-industrys-most-powerful-ai
- AWS, Modernizing Clinical Trials: Digital Technologies and the Cloud, Available: https://pages.awscloud.com/rs/112-tzm-766/images/aws_clinicaltrials_whitepaper_final.pdf
About The Authors:
 Kannan Ramachandran is a category specialist at Beroe Inc., a global procurement intelligence firm that partners with over 10,000 companies worldwide, including a majority of the Fortune 500. With deep expertise in cloud computing and data center infrastructure, Kannan advises enterprises on strategic sourcing, cost optimization, and digital transformation. His work is rooted in data-driven research and market intelligence, helping organizations make informed decisions in a rapidly evolving tech landscape.
Kannan Ramachandran is a category specialist at Beroe Inc., a global procurement intelligence firm that partners with over 10,000 companies worldwide, including a majority of the Fortune 500. With deep expertise in cloud computing and data center infrastructure, Kannan advises enterprises on strategic sourcing, cost optimization, and digital transformation. His work is rooted in data-driven research and market intelligence, helping organizations make informed decisions in a rapidly evolving tech landscape.
 Mathini Ilancheran is a research manager, R&D, at Beroe Inc. She is an expert in procurement intelligence and industry analysis, specializing in strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. Mathini has authored 37+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
Mathini Ilancheran is a research manager, R&D, at Beroe Inc. She is an expert in procurement intelligence and industry analysis, specializing in strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. Mathini has authored 37+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
