eClinical Solutions Market To Double In 5 Years, As Sponsors Prioritize Efficiency
By Tushar Genji, assistant manager, and Shankar Govindrao Kadam, team lead, MarketsandMarkets
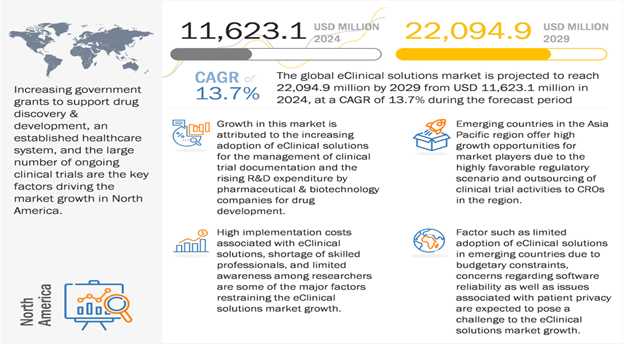
The global eClinical solutions market is projected to reach $22.1 billion in 2029 from $11.6 billion in 2024 at a CAGR of 13.7%. eClinical solutions are software/platforms that replace the paper-driven clinical research model to streamline clinical trial data collection, data transmission, and monitoring. They also offer advanced tools for planning and managing the clinical trial process, making the process faster, reducing risk, and involving fewer resources. These solutions optimize the product development and commercialization process for pharmaceutical, biopharmaceutical, and medical device companies, as well as CROs and service providers.
Top Reasons For Using eClinical Solutions
Amid regulatory compliance pressures, pharmaceutical and biopharmaceutical companies are seeking ways to decrease the cost and manage the risk of clinical trials, which in turn is increasing the demand for eClinical solutions. The rising operational costs and regulatory requirements associated with clinical research studies, increasing R&D expenditure on drug development by pharma-biotech companies, favorable government support, and funding for clinical trials fuel this market's growth globally. The rising need for improved data standardization and the growing adoption of novel software solutions in clinical research are some of the major driving factors for the global eClinical solutions market.
Emerging Technologies In Clinical Research
In recent years, eClinical solutions and automated systems have been replacing traditional manual processes, significantly reducing the time and effort required to handle clinical trials. These technologies can help streamline operations, improve data accuracy, optimize clinical trial efficiency, ensure regulatory compliance, and accelerate clinical trial timelines.
AI-Enabled and Integrated Platforms
Al-enabled and integrated eClinical platforms can improve data management, analysis, and even decision-making during the clinical phase or product development. Al integration into eClinical platforms can facilitate the automation of routine tasks, analyze large data sets for patterns and insights, predict outcomes, and optimize trial designs. Such integration will bring improvement in efficiency, accuracy, and the overall effectiveness of clinical trials, thus helping to quicken the development of new treatments and therapies.
Clinical Data Integration
Clinical data integration involves collecting organized data from clinical trials and healthcare settings and using advanced analysis techniques to drive meaningful insights. Data integration combines information from patient records, laboratory results, wearable devices, and more on unified platforms that enable a complete analysis by researchers and clinicians. It helps researchers discover patterns, trends, and predictive models, further influencing treatment decisions, optimizing trial designs, and personalizing treatment. With clinical data integration, real-time monitoring helps with proactive intervention and strategy adjustment given its continuous data analysis.

eClinical Solutions Market: Ecosystem Analysis
Complementary Technologies In Clinical Research
Several supporting technologies enhance efficiency, accuracy, and overall effectiveness in the evolving eClinical solution landscape. These technologies integrate seamlessly with core clinical trial processes to provide a comprehensive solution for researchers. Here are some of the complementary technologies in eClinical solutions.
Cloud Computing
Cloud computing supports clinical solutions by revolutionizing data management and analysis. Cloud platforms provide secure and scalable environments for storing and sharing clinical data, enabling seamless collaboration amongst research teams across geographical boundaries.
EDC, CTMS, and Other Analytical Tools
Clinical trials generate massive amounts of data, including patient demographics, medical histories, and treatment responses. eClinical solutions such as electronic data capture and clinical trial management systems ensure the collection, storage, and organization of these large amounts of data. Big Data analytics tools help identify patterns, trends, and missing information that traditional methods cannot fully capture.
Al analytics extracts insights from complex clinical trial data. It forecasts timelines for clinical trial completion, reducing the burden of manual data review. It also predicts patient retention rates and improves data quality for enhanced decision-making, ultimately helping to accelerate the drug development process and improve the patient experience.
Clinical Research-Adjacent Technologies
In the dynamic field of eClinical solutions, several adjacent technologies complement the clinical trial processes, enhancing overall healthcare delivery and operational efficiency. While not directly part of the clinical process, these technologies significantly improve the ecosystem in which the trial operates. The following are some of the key adjacent technologies in eClinical solutions.
Electronic Health Records
Integrating electronic health records (EHRs) provides a competitive advantage to eClinical platforms. EHRs contain valuable patient information such as medical histories, treatment plans, allergies, and lab results, which are essential for efficient clinical trials. Thus, a researcher can access a vast repository of real-world patient data, accelerate patient recruitment, enhance data quality, and improve trial efficiency.
Real-World Data
Using eClinical platforms to access real-world data (RWD) from real-world sources such as claims databases, a researcher can obtain a full picture of patient populations, illness development, and treatment outcomes. This facilitates faster medication development, better trial design, and more effective patient recruitment. RWD also makes it easier to assess the efficacy of medications in practical situations, closing the gap between clinical trials and actual patient care.
Bioinformatics
In eClinical solutions, bioinformatics refers to the efficient management and analysis of biological and clinical data using computer tools and techniques. Advanced analytics such as variant analysis, genome sequencing, and biomarker discovery are made possible by bioinformatics algorithms to optimize clinical trial designs and find possible treatment targets. eClinical solutions facilitate the development of targeted medicines, provide accurate patient categorization based on genetic profiles, and faster drug discovery. This integration of bioinformatics improves the efficacy and efficiency of clinical research, leading to better patient results.
Increasing R&D Expenditure To Drive Market
Pharmaceutical companies primarily use eClinical solutions to increase the efficiency of clinical trials and reduce overall cost and time in drug development. These benefits have increased eClinical solution adoption and driven growth in the market. Pharmaceutical companies’ increasing R&D expenditure is expected to continue to drive demand for these IT solutions. Sponsors should consider increased clinical trial complexity, larger clinical trial sizes, growing focus on targeting chronic and degenerative diseases, and higher failure rates of new medications in earlier phases of clinical trials as reasons to invest in eClinical solutions. Doing so might also reduce costs.
However, small and medium-sized pharmaceutical companies are increasingly outsourcing their research and data management activities to CROs due to budgetary constraints and the high cost of software solutions. Those finding themselves in this position may decide not to purchase eClinical solutions. In turn, this may restrict the growth of the global eClinical solutions market.
Final Thoughts
To meet stringent regulatory requirements, researchers focus on maintaining and improving the quality of clinical trial procedures. Yet, some trials experience inadequate record keeping, protocol deviation, insufficient monitoring, failure to follow the investigational plan and signed investigator statement/agreement, and inadequate subject protection. eClinical software solutions help researchers overcome obstacles and avoid missteps, thereby increasing prospects for early regulatory approval and product commercialization. Therefore, biopharmaceutical companies and research organizations are increasingly adopting healthcare IT solutions to improve and simplify their research/clinical trial processes.
 About The Authors:
About The Authors:
Tushar Genji, assistant manager with MarketsandMarkets, has 8+ years of experience creating revenue impact for clients’ businesses by providing actionable insights. In his current role, he specializes in market research and forecasting for the syndicate and consults on studies related to healthcare, life sciences, and healthcare IT, delivering insights into market entry, growth, and go-to-market strategy, as well as advising clients on digital strategy, road map, and competitive analysis.
 Shankar Govindrao Kadam is a team lead for MarketsandMarkets. He has 5+ years of experience in market research and qualitative/quantitative analysis for the healthcare IT sector.
Shankar Govindrao Kadam is a team lead for MarketsandMarkets. He has 5+ years of experience in market research and qualitative/quantitative analysis for the healthcare IT sector.
