Castor-eCOA Clinical Trial Technology

1,735 eCOA & ePRO Studies Completed
eCOA and ePRO with Enrollment, eConsent, Payments and EDC integrated. Our services cover build, licensing and 24/7 support.
95%+ Patient Compliance Rate
4-8 Weeks to Deploy
190+ Validated Instruments
Technology Built for Disease Complexity
From rare disease natural history to oncology trials, our platform adapts to your therapeutic area's unique requirements
Oncology
200+ studies powered by pre-built templates (EORTC QLQ-C30, FACT-G), real-time AE safety notifications, and integrated MedDRA/WHODrug coding.
CNS/Mental Health
150+ studies using event-driven scheduling for PHQ-9 & HAM-D, with dynamic diary triggers based on patient input and real-time alerts for missed entries.
Rare Diseases
30+ studies supported with low-burden protocols featuring integrated Televisits, remote eConsent with Legally Authorized Representative (LAR) workflows, and a BYOD-first approach to reduce travel.
Cardiology
Direct device integration via open API captures real-time wearable data, with large file upload support (up to 2GB) for storing crucial medical imaging alongside the patient record.
90+ Countries
1,735+ eCOA Studies
95%+ Compliance Rate
24/7 Support in 80+ Languages
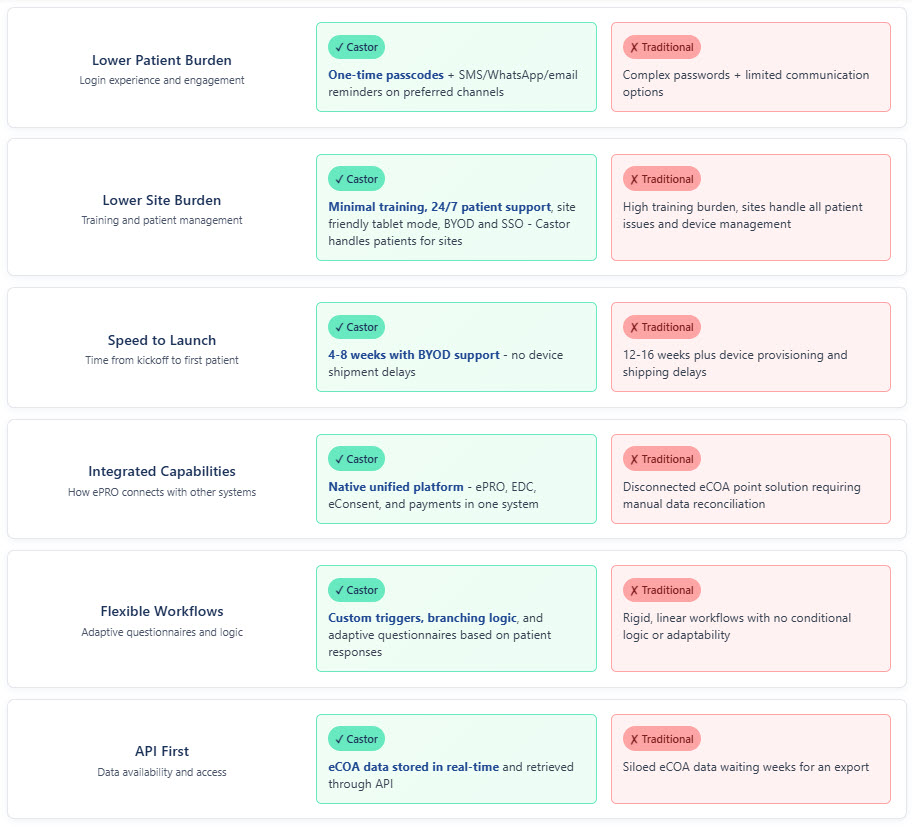
eCOA Without Burden
Frictionless Patient Experience
- One-time passcodes, no passwords to remember
- Multi-language support (80+ languages)
- Works on any device - smartphone, tablet, or computer
- Offline capability for remote areas
Reduce Site Burden Dramatically
- Site friendly tablet mode for efficient onsite patient data collection
- Minimal training required with intuitive interfaces
- 24/7 patient support - Castor handles patient issues for you
- BYOD and SSO capabilities for streamlined operations
- Real-time compliance monitoring dashboards
- Automated reminders reduce follow-ups by 40%
Streamlined eCOA & ePRO Study Management System
- API-first integration with any clinical outcome assessment system
- Real-time ePRO data capture and monitoring software
- Integrated patient payments and reimbursements platform
- Single eCOA solution for EDC, ePRO, and eConsent
Full-Service eCOA & ePRO Solutions Implementation
Expert team handles complexity, you focus on research
Scale Licensing
Strong relationships with copyright holders to secure agreements. Expert coordination of language reviews and scale adaptations.
Translations
Validated linguistic adaptations across 80+ languages. Vast network of translation vendors ensuring scientific validity.
Direct-to-Patient Support
24/7 multilingual helpdesk with global toll-free numbers. Includes device provisioning management when needed. Response times under 1 hour with real-time issue escalation.
eCOA Library
120+ pre-validated instruments for Oncology, Cardiology, and other therapeutic areas. Quick deployment with proven quality.
Why Traditional eCOA Systems Fail
Not all ePRO solutions are equal. Traditional clinical outcome assessment systems burden sites and frustrate patients.